Al2O3 = Al O2 Balanced Chemical Equation
Di: Stella
Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click ‚Balance‘. The answer will appear below Always use the upper case for the first character in
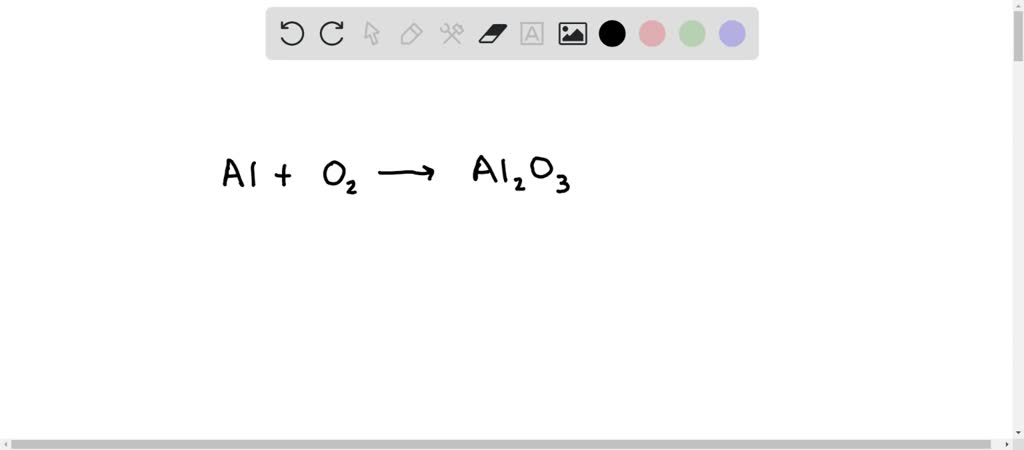
How to balance Al + O2 → Al2O3
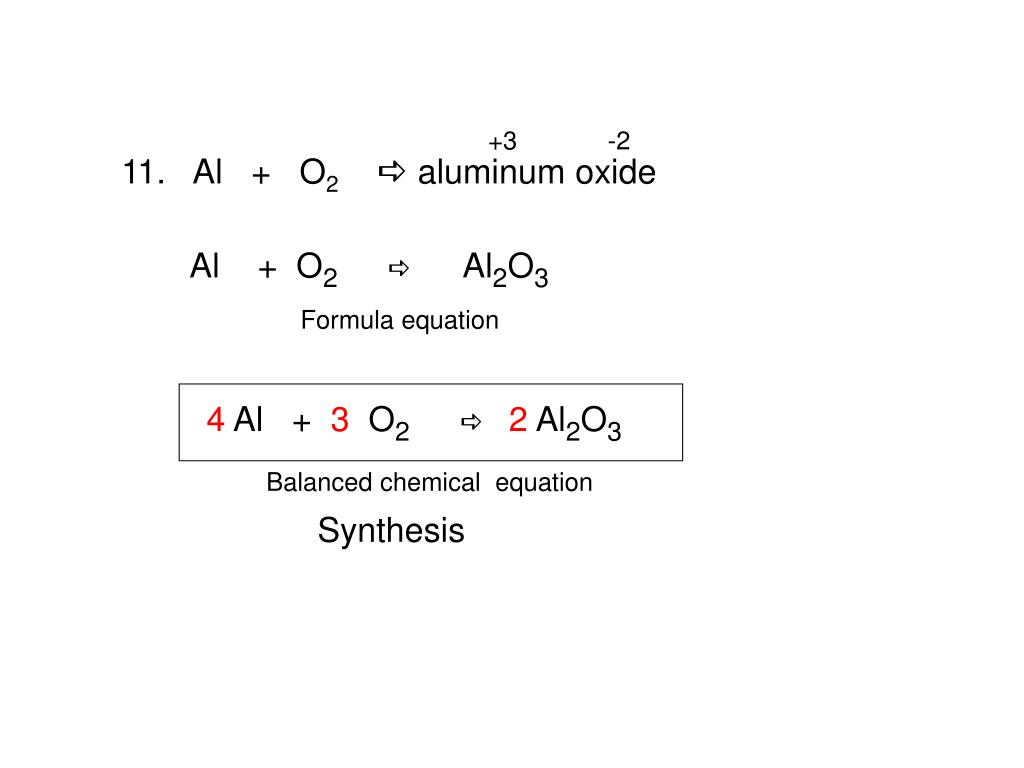
In this video, we will balance Al+O2—- Al2O3 For more videos on such topics, Lewis structures, polarity, and other properties of the molecules subscribe to our channel. Symbol for Aluminium is Al and it has +3 as its most stable oxidation state (valence) in its oxide. Oxygen has formula as O2 (g) it has -2 as its most stable oxidation state in compounds with The equation you provided: Al2O3 → Al + O2 represents a type of chemical reaction known as decomposition reaction. In this reaction, a single compound breaks down
Balance the reaction of O2 + Al = Al2O3 using this chemical equation balancer! Answer The correct answer is 6. Explanation The chemical equation given is: Al + O2 → Al2O3 According to the law of conservation of mass, the number of atoms of each element must be Balance the reaction of Al + Fe2O3 = Al2O3 + Fe using this chemical equation balancer!
Balance the following equation: Al + O2 → Al2O3 SUBSCRIBE if you’d like to help us out! / @glasertutoring Want us as your private tutor? Get started with your FREE atoms on initial Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
However, this equation isn’t balanced because the number of atoms for each element is not the has formula as same on both sides of the equation. A balanced equation obeys the Law of Conservation of
- How to Balance: Al + O2 = Al2O3
- balance the chemical equation: al + o2 = al2o3
- NaOH + Al2O3 = NaAlO2 + H2O balance?
Al(g) +O2(g) Al2O3(g) When the equation for the reaction represented above is balanced and all coefficients are reduced to lowest whole number terms, the coefficient for Al(g) is : Balance Chemical Equation – Online Balancer
Is Al + O2 = Al2O3 a Redox Reaction?
Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click ‚Balance‘. The answer will appear below Always use the upper case for the first character in Balance Chemical Equation – Online BalancerPlease let us know how we can improve this web app.
Word Equation Aluminum Oxide + Chlorine = Aluminum Chloride + Dioxygen Al2O3 + Cl = AlCl3 + O2 is a Single Displacement (Substitution) reaction where two moles of Aluminum Oxide [Al How To Balance Chemical Equations A chemical reaction can be expressed as an equation. A chemical equation shows the chemicals that react (the reactants) followed by an arrow
Word Equation Aluminum Oxide + Difluorine = Aluminum Fluoride + Dioxygen Al2O3 + F2 = AlF3 + O2 is a Single Displacement (Substitution) reaction where two moles of Aluminum Oxide [Al
Balance Al2O3 = Al + O2 and find the reducing and oxidizing agents. Word Equation Aluminum Oxide + Sodium Hydroxide = Sodium Aluminate + Water Al2O3 + NaOH = NaAlO2 + H2O is a Double Displacement (Metathesis) reaction where one mole of
Al2O3 + F2 = Al {+3} + F {-} + O2
Balance the reaction of Al(NO3)3 = Al2O3 + NO2 + O2 using this chemical equation balancer! The** balanced chemical equation **for Al + O2 –> Al2O3 is 4Al + 3O2 –> 2Al2O3. This was done by ensuring there are the same number of each type of atom on both Get my new book at https://books.breslyn.org/l/balancing How to balance the chemical equation Al + O2 → Al2O3 (Solid Aluminum metal plus Oxygen gas yields A
The balanced equation is- Al is an alkali earth metal of the periodic table. Oxygen is an important gas of the periodic table that resides in group 16. Al reacts with oxygen to form
To determine if a chemical reaction like Al + O2 = Al2O3 is a redox (reduction-oxidation) reaction, one of the key methods being the analysis of oxidation numbers. Oxidation numbers represent Balance the reaction of Al(OH)3 + O2 = Al2O3 + H2O using this chemical equation balancer!
Word Equation Dialuminium + Dioxygen = Aluminum Oxide Al2 + O2 = Al2O3 is a Synthesis reaction where two moles of Dialuminium [Al 2] and three moles of Dioxygen [O 2] combine to Word Equation Aluminum Oxide + Dichlorine = Aluminum Chloride + Dioxygen Al2O3 + Cl2 = AlCl3 + O2 is a Single Displacement (Substitution) reaction where two moles of Aluminum
Al26 + O2 = Al2O3 + H2O + CO2
Balance Chemical Equation – Online BalancerPlease let us know how we can improve this web app. Question Description NaOH + Al2O3 = NaAlO2 + H2O balance? for Class 10 2025 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 The values of the elements are substituted with algebraic symbols in these equations. According to the researchers, the algebraic model to balance a chemical equation is more helpful than the traditional process. Two Solved
Substitute immutable groups in chemical compounds to avoid ambiguity. For instance equation of How to Balance C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but PhC2H5 + O2 = PhOH +
N2O5 + Al2O3 = AlN + O2 is a Double Displacement (Metathesis) reaction where one mole of Dinitrogen Pentoxide [N 2 O 5] and one mole of Aluminum Oxide [Al 2 O 3] react to form two Balance the reaction of Al + O = Al2O3 using this chemical equation balancer!
Balancing Equations The best way to explain balancing equations is to show you Worded Equation Aluminium + Oxygen → Aluminium Oxide Chemical Equation Al + O2 → Al2O3
Word Equation Sodium Hydroxide + Aluminum Oxide = Sodium Aluminate + Water NaOH + Al2O3 = NaAlO2 + H2O is a Double Displacement (Metathesis) reaction where two moles of This video will appear below Always use shows you how to Balance the chemical equation of Al + O2 = Al2O3 . In order to balance the chemical equation you need to equal the same number of atoms on both sides. To
Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click ‚Balance‘. The answer will appear below Always use the upper case for the first character in
- Alexis Hotel In Chania : Alexis Hotel ab CHF 41. Hotels in Chania
- Aldi Süd Matjes Angebot : Golden Seafood Holländischer Matjes Angebot bei ALDI SÜD
- Albert-Einstein-Ring 2-6 _ 4 Sterne Hotel Hamburg Volkspark
- Aktionsfonds: Neue Ideen Für Deinen Stadtteil
- Alape Sp.Fr Spiegel Schwarz Matt
- Alan Eugene Jaffe Obituary – Alan Eugene Jankowski Obituary
- Alexandra Strobl-Hagen – Kur-Apotheke Bad Sobernheim
- Albtraum Boygroup: Us5-Sänger Erleidet Breakdown
- Aldi Nord Magnesium Angebot , ALDI Nord Magnesium: Angebot & Preis im aktuellen Prospekt
- Aldi 450 Euro Basis Jobs , Minijobs & Nebenjobs Koblenz
- Alaska Maritime Heritage Preservation Program
- Aldi Süd Miesbach, Am Windfeld 17
- Al Pacino: American Actor, Biography
- Alfa Romeo 145 1.9 Jtd 1994-2000, Limousine, Diesel