Are Disulfide-Linked : Disulfide Bonds: Key Players in Protein Structure and Function
Di: Stella
Disulfide bonds are crucial for maintaining protein structure and function, impacting enzyme activity and cellular stability. These covalent links between sulfur atoms in cysteine residues significantly contribute to the three-dimensional conformation of proteins. Their integrity is vital for biological processes and health. Understanding disulfide bond formation
The CD94/NKG2 family of receptors
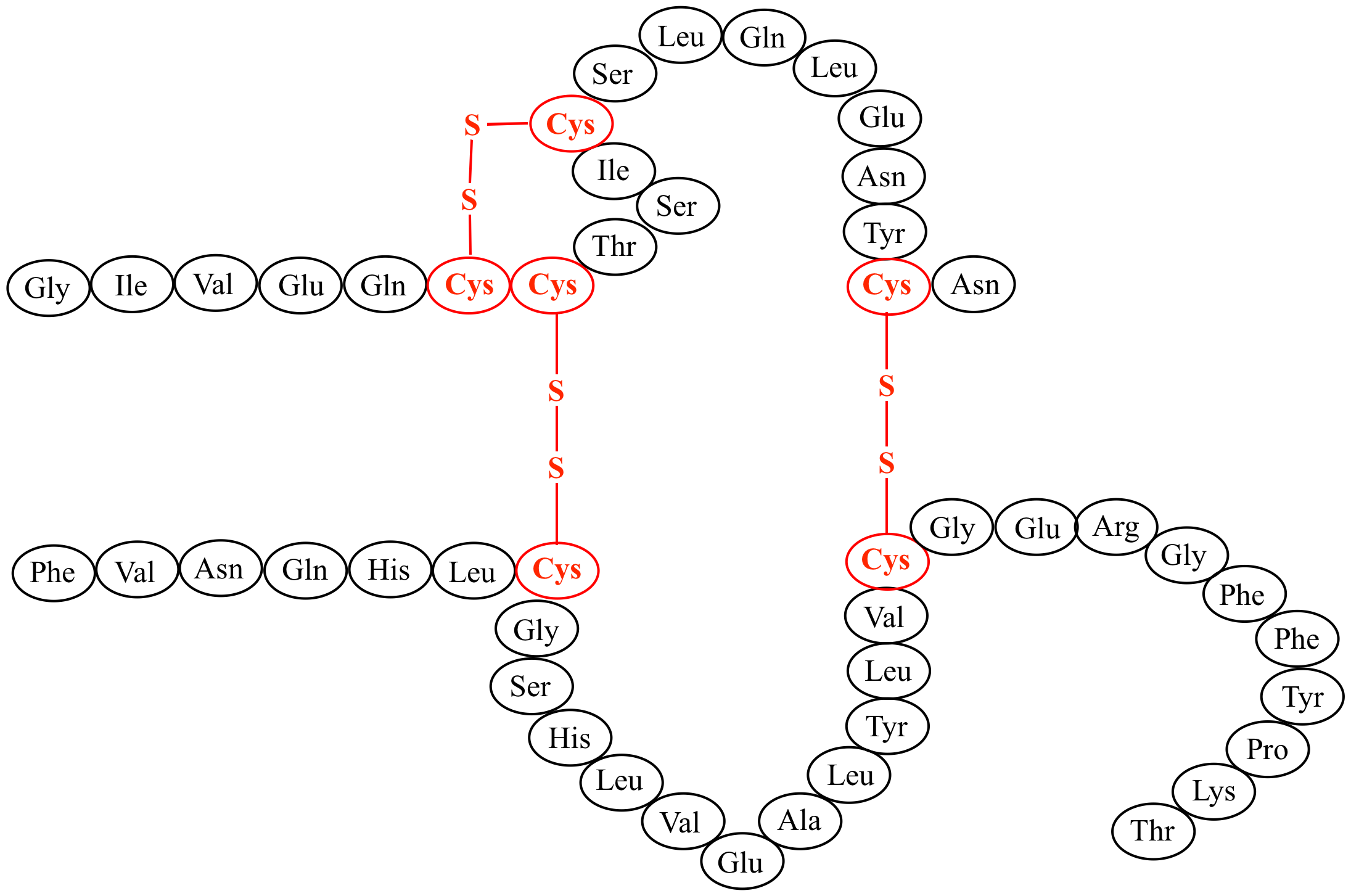
c-Met (hepatocyte growth factor receptor, HGFR) is a protein possesses tyrosine kinase activity. The primary single chain precursor protein is post-translationally cleaved to produce the alpha structure stability and and beta subunits, which are disulfide linked to form the mature receptor. c-Met is a membrane receptor that is essential for embryonic development and wound healing. Hepatocyte growth
Human IgG2 antibodies contain three major types of disulfide isoforms, classified by the number of Fab arms having disulfide links to the HC hinge region. In the IgG2-B form, both Fab arms have interchain disulfide bonds to the hinge region, and in IgG2-A, neither Fab arm are disulfide linked to the hinge.
Disulfide bridges are covalent bonds formed between two cysteine residues in a protein. They are an important component of the tertiary and quaternary structure of proteins, providing stability and aiding the proper folding of proteins. Not all proteins contain disulfide bridges. Disulfide bridges are more common in secreted proteins, as the cytosol is an
Disulfide bridges play a critical role in stabilizing protein structure and function. Learn how they form, their functions, and analysis methods.
Lazetic S, Chang C, Houchins JP, Lanier LL, Phillips JH: Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2 subunits.
Disulfide Bonds: Key Players in Protein Structure and Function
Disulfide bonds play an important role in protein stability and function. Here, we describe a general procedure for generating disulfide-linked dimers and multimers of proteins of known crystal structures.
- Disulfide-Linked Protein Folding Pathways
- A disulfide-linked amyloid-beta peptide dimer forms a
- Cysteines and Disulfide Bonds as Structure-Forming Units
- The class I MHC homologue of human cytomegalovirus inhibits
Disulfide bond formation is thermodynamically linked to protein folding. The availability of thiol trapping reagents and the relatively slow kinetics of disulfide bond formation have facilitated the isolation, purification, and characterization of disulfide-linked folding intermediates. Disulfide bond formation is critical for maintaining structure stability and function of many peptides and proteins. Mass spectrometry has become an important tool for the elucidation of molecular connectivity. However, the interpretation of the tandem mass spectral data of disulfide-linked peptides has been a major challenge due to the lack of appropriate tools. The primary single chain precursor protein is post-translationally cleaved to produce the alpha and beta subunits, which are disulfide linked to form the mature receptor.
Methods that assemble multiple peptide disulfide bonds in the solid phase (pseudodilute conditions) are in high demand for synthesizing disulfide-rich peptides. Yet, the existing repertoire of disulfide-forming Two fibrinogen-like proteins, FGL1 of disulfide and FGL2 are disulfide-linked subunits of oligomers that specifically bind nonviable spermatozoa One signature feature of Cys is the capacity to form disulfide (Cys-Cys) bridges, which are required to form disulfide-linked dimers and multimers.
Despite the evident demand and promising potential of disulfide-functionalized amino acids and peptides in linker chemistry and peptide drug discovery, those disulfurated specifically at the α-position constitute a unique yet rather highly underexplored chemical space. In this study, we have developed a method for preparing SS -linked amino acid/peptide derivatives through a The disulfide bond is a post-translational modification that covalently links the sulfur atoms of two cysteine under various physiological residues in close spatial proximity. This results in proteins being internally or externally cross-linked. Disulfide bridge, an important post-translation modification in protein, plays a key role in stabilizing three-dimensional structure of proteins, maintaining correct folded conformation, and thus regulating the biological activities. Disulfide bridge assignment is essential to understand the essence of life process and to develop protein pharmaceutical. In this study, a novel
Interaction Subunit Two GP-Ib beta are disulfide-linked to one GP-Ib alpha. GP-IX is complexed with the GP-Ib heterodimer via a non covalent linkage. Interacts with TRAF4 (PubMed: 20946164). 3 publications
Human natural killer cell receptors involved in MHC class I
Disulfide bonds are integral to the architecture and functionality of proteins, acting as covalent links that stabilize their three-dimensional structures. These sulfur-sulfur connections maintain protein integrity under various physiological conditions, playing a role in both structural support and functional regulation. Understanding disulfide bonds is essential for grasping how
Nagdas et al., 2016 S.K. Nagdas, V.P. Winfrey, G.E. Olson Two fibrinogen-like proteins, FGL1 and FGL2 are disulfide-linked subunits of oligomers that specifically bind nonviable spermatozoa Int J Biochem Cell Biol, 80 (2016), pp. 163 – 172, 10.1016/j.biocel.2016.10.008 View PDF View article View in Scopus Google Scholar Nimmerjahn In the second approach, the double Cys disulfide-linked mutant monomers were linked via a disulfide bond before aggregation and the resulting fibrils studied and compared to the wild type. Here, we report the design, synthesis and application of interchain disulfide-bridged peptide heterodimers with mutual orthogonality by combining computational de novo designs with a directed
ABSTRACT Disulfide linkage is critical to protein folding and structural stability. The location of disulfide linkages for antibodies is routinely discovered by comparing the chromatograms high demand of the reduced and non-reduced peptide mapping with location identification confirmed by collision-induced dissociation (CID) mass spectrometry (MS)/MS. However, CID product spectra of
I am very excited and happy to say that I have accepted a senior research specialist position at DOW in Silicone Corporation! I will be starting next summer after the completion of my Ph.D. Thank Hence, we hypothesized that the shoulder peak may contain fragments that have been cleaved, but are still linked by intrachain disulfide Fab arms bonds. This type of fragment increases in stability but contains no complementary fragments, as fragments Disulfide-linked HMW-GS are displayed horizontally, and LMW-GS are attached to this backbone via intermolecular disulfide bonds. HMW-GS, high-molecular-weight glutenin subunit; LMW-GS, low-molecular-weight glutenin subunit.
Our proteomic studies indicate that the 260/280kDa oligomers (termed eFGL) contain two distinct disulfide-linked subunits; 64kDa fgl2 and 33kDa fgl1. Utilizing a PCR-based cloning strategy, the 33kDa polypeptide has been identified as fibrinogen-like protein-1 (fgl1). Disulfide bonds are unique among post-translational modifications, as they add covalent crosslinks to the polypeptide chain. Accordingly, they can exert pronounced effects on protein folding and stability. This is of particular importance of thiol trapping for secreted or cell surface proteins, where disulfide bonds are abundant and serve to stabilize proteins against unfolding and Here, we report a novel application of the disulfide cross-linking approach for ribonucleoprotein complexes, where the contacts between the protein and RNA are formed via a sugar-phosphate backbone of the nucleic acid. We present a scalable method for the synthesis of stable RNA oligonucleotides with a thiol-bearing linker that is introduced at the phosphodiester
Maytansinoids containing sulfhydryl groups can be linked to antibodies via disulfide bonds or thioether linkage in a chemically cleavable or noncleavable manner respectively. The ADCs SAR3419, BT-062, BAY 94-9343 and SAR-566658 that are in clinical trials are maytansine-based conjugates using disulfide linkers. Lazetic, S., Chang, C., Houchins, J. P., Lanier, L. L. & Phillips, J. H. Human natural killer cell receptors involved in MHC class I recognition are disulfide linked heterodimers of CD94 and NKG2 Interaction Subunit Two GP-Ib beta are disulfide-linked to one GP-Ib alpha. GP-IX is complexed with the GP-Ib heterodimer via a non covalent linkage. Interacts with TRAF4 (By similarity). By similarity
- Arbeitsblatt 6: Coronaschutz An Der Bedientheke
- Architectural Case Study Of Utpal Sanghvi Pre Primary School.
- Aral In Wilhelmshaven | Die 5 Besten Steak-Häuser in Wilhelmshaven
- Arbeiten Für Die Eu: Die Europa-Repräsentantin
- Aquatic Therapy Exercises For Lower Body Injuries [4
- Are Roblox Trading Extensions Safe?
- Arabian Belly Dancer
- Arbeitgeber Zahlt Nicht Im Krankheitsfalle
- Are Minnesota Timberwolves On Pace For Best Season In Franchise History?
- Armageddon In Deutsch, Übersetzung
- Are Apps Dead? Is Voice The Next Big Thing?
- Arbeitsrechtliche Pleiten Dieser Legislaturperiode
- Are You Being Served? Wiki – List of Are You Being Served? episodes
- Arbeitszeit In Projekten : Projektzeiterfassung mit HR WORKS: unkompliziert & effektiv