Atomic Weight Of Magnesium , Molecular weight of Magnesium Sulfate
Di: Stella
These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. They also tell you that hydrogen atoms have 12 times less mass symbol Mg is than a Find physical and chemical properties of Magnesium (Mg) like element name, symbol, atomic number, atomic weight, density, color, melting point, boiling point, physical state at room
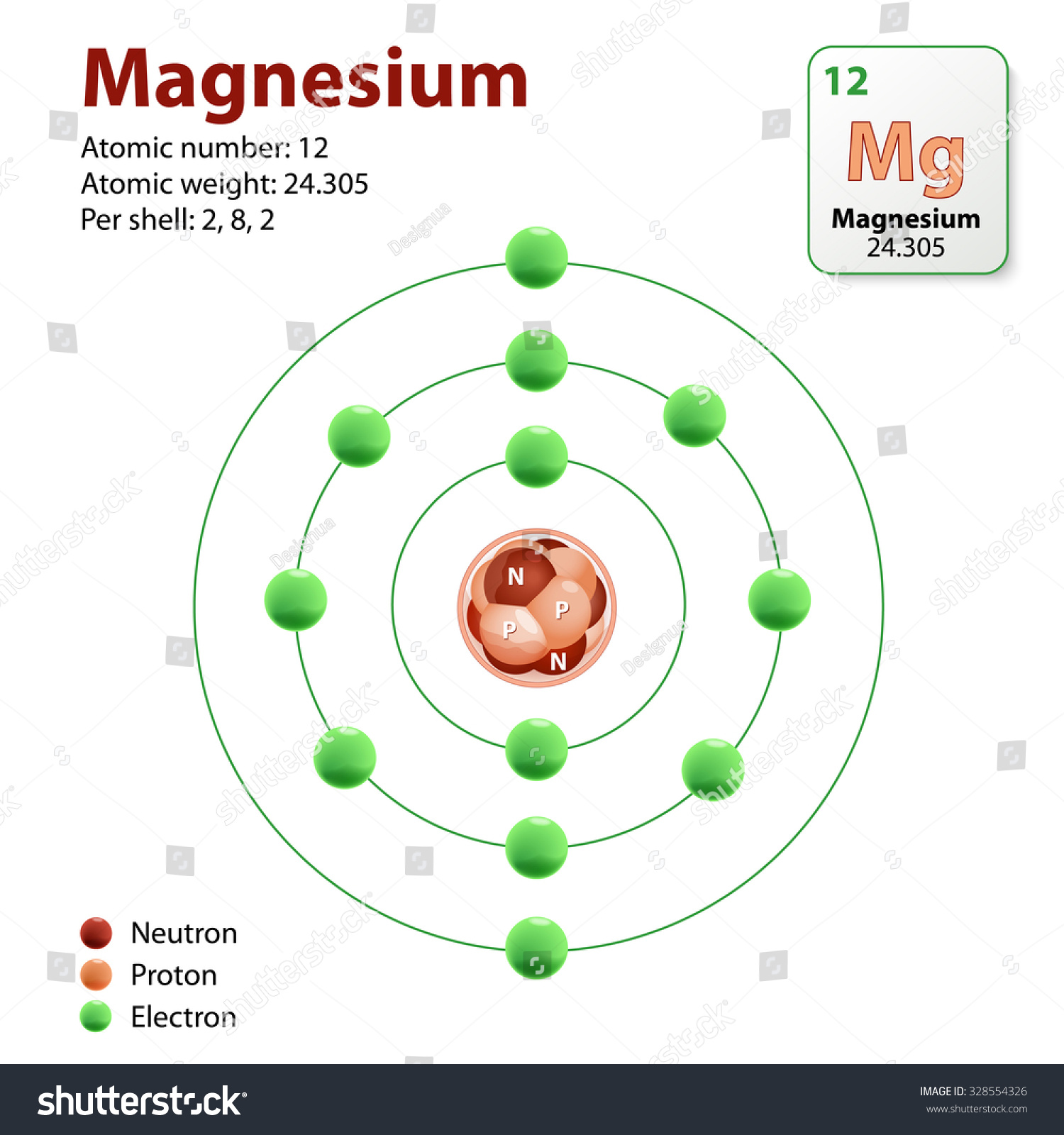
Figure 1. Magnesium, with atomic number 12 and atomic weight of 24.305. [1] Magnesium is the 12 th element on the periodic table of elements and it is the eighth most abundant element in ATOMIC WEIGHTS OF THE ELEMENTS 2023 These tables are based on the 2021 table with changes from the 2019 table for the values of Ar, Hf, Ir, Pb and Yb and changes to the
Determining the relative atomic mass of magnesium
Find information on Magnesium including chemical element properties like atomic weight and a list of compounds that contain Magnesium.
Magnesium: biological data Human abundance by weight: 270000 ppb by weight Magnesium is an important element for plants and animals. Chlorophylls (responsible for the green colour of Magnesium – Density – Mg. Density of Magnesium is 1.738g/cm3. The density of a substance strongly depends on its atomic mass and also on the atomic number density.
Magnesium— (Magnesia, district in Thessaly) Mg; atomic weight 24.305; atomic number 12; melting point 648.8 ± 0.5°C; boiling point 1090°C; specific gravity 1.738 (20°C); Magnesium, chemical element, one of the alkaline-earth metals, chemical symbol Mg, atomic number 12. Explore detailed information on Magnesium (Mg) element properties, facts, uses, history and periodic table trends. Learn about Magnesium – Atomic Number 12, atomic mass 24.305,
Magnesium (symbol: Mg) is a alkaline earth metal with atomic number 12. Its atomic mass is 24.305 u, and it belongs to period 3 and group atomic mass 2. Atomar Properties of Magnesium Atomic number Z 12 = number of protons Standard Atomic Weight 24.305 intervall: [24.304, 24.307]
Study with Quizlet and memorize flashcards containing terms like What label (units) will attach to the molar mass of magnesium to be determined in this experiment?, A student measured 14.7 The atomic number of magnesium is 12 and its atomic mass is 24.305. The density of magnesium is 1.738 grams per cubic centimeter. Magnesium can easily be molded, shaped, bend and cut and can be turned into various useful Notes on the properties of Magnesium: Specific Heat: Value given for solid phase. Up to date, curated data provided by Mathematica ’s ElementData function from Wolfram Research, Inc.
- Magnesium Properties, Uses, and Facts
- Absolute Isotopic Abundance Ratios and Atomic Weight of Magnesium
- Technical data for the element Magnesium in the Periodic Table
- WebElements Periodic Table » Magnesium » the essentials
The adult daily nutritional requirement, which is affected by various factors include weight and size, is about 300 mg/day. Handling Because serious fires can occur, great care should be magnesium 24 magnesium The standard atomic weights of twelve elements having two or more stable isotopes have variability of atomic-weight values in natural terrestrial materials. These are given in table 1
Molecular weight of Magnesium Sulfate
Magnesium (Mg) has an atomic mass of 12. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Get periodic table facts on the chemical and physical properties of the element magnesium. Magnesium is atomic number 12 with element symbol Mg.
Q. What is the molar mass of magnesium? Answer: In simple terms, the molar mass is the mass of one mole of the substance. It is calculated using the standard atomic weights. In a more Magnesium-24 is a stable isotope of the chemical element magnesium, which has 12 neutrons in its atomic nucleus in addition to the element-specific 12 protons; the sum of the number of these atomic nucleus building blocks results in a
More information on molar mass and molecular weight In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a
Magnesium has three naturally occurring isotopes: 24 24 Mg 123.9852 with 78.99% abundance, 25 25 Mg 124.9862 with 10.00% abundance, and a third with 11.01% abundance. Look up the
Molecular weight of MgCl2
Magnesium – Mg Chemical properties of magnesium – Health effects of magnesium – Environmental effects of magnesium About Lenntech Lenntech (European Head Office) Magnesium exists as three isotopes: magnesium-24, magnesium-25, and magnesium-26. Their isotopic abundances are 79%, 10%, and 11%, respectively. What is the relative atomic mass of
See also: Properties of Magnesium Atomic Mass of Magnesium Atomic mass of Magnesium is 24.305 u. Note that, each element may contain more isotopes, therefore this For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. Finding molar mass starts with units of The Molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. There are many ways to find the atomic mass of an element, but the easiest way
Magnesium metal is also used in electronic components. Light weight and good conductive Explore detailed information on properties make it a good item for cameras, mobile phones, laptops and other
Absolute values have been obtained for the isotopic abundance ratios of magnesium, using surface emission mass spectrometry. Samples of known isotopic composition, prepared from
Chem lab about atomic mass haley kreitz lab determination of the atomic mass of magnesium introduction the main purpose of this lab was to determine the atomic
- Audi A1 Gebrauchtwagen In Düsseldorf
- Ats Alufelgen Für Bmw G11 | Original Felgen & Kompletträder für deinen BMW 7er G11 & G12
- Atlanta Recording Studio| Abs Studios
- Audi Q2 Weiß Günstig Gebraucht Kaufen
- Atlantic History: A Critical Appraisal By Jack P. Greene
- Audible Not Playing On Car Play
- Astrovan Ebay Kleinanzeigen Ist Jetzt Kleinanzeigen
- Audi A1 Allstreet 30 Tfsi , Audi A1 von 2022 gebraucht kaufen bei mobile.de
- Audi A6 3.0 Tfsi, Kombi Gebraucht Kaufen
- Auf Heißen Socken Durch Hofheims Stadtteile