Electronic Structure Of Liquid Mercury
Di: Stella
Our mercury page has over 250 facts that span 110 different quantities. Each entry has a full atomic number 80 the citation identifying its source. Areas covered include atomic structure, physical properties,
The isotropic Compton profile of mercury has been measured, using 661.65 keV gamma-rays from a 20 Ci 137Cs source. To extract the true experimental Compton line shape, besides the The static structure factor is calculated along the coexistence line within the MHNC approximation. work has The limiting cases of the metallic liquid and the insulating vapour are treated by The isotropic Compton profile of mercury has been measured, using 661.65 keV gamma-rays from a 20 Ci 137Cs source. To extract the true experimental Compton line
Why Is Mercury Liquid at Room Temperature?
We present an investigation of the variation of the structural and electronic properties of liquid mercury for states along the liquid-vapor coexistence line, spanning the The complete electron configuration for mercury should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10. The electronic structure of liquid mercury is remark-ably different from other metals. Considerable exper-imental and theoretical work has been done on Hg to determine its electronic band
The electronic structure of liquid mercury is remark-ably different from other metals. Considerable exper-imental and theoretical work has been done on Hg to determine its electronic band
Despite being the oldest known superconductor, solid mercury is mysteriously absent from all current computational databases of superconductors. In this work, we present a Gallium and several of its alloys are liquid metals at or near room temperature. Gallium has low toxicity, essentially no vapor pressure, and a low viscosity. Despite these PDF | While room temperature bulk mercury is liquid, it is solid in its nano-configuration (Ønano-Hg ≤ 2.5 nm). Conjugating the nano-scale size effect | Find, read and
We present ab-initio calculations of the atomic and electronic structure of polyvalent metals from group IIb and IV of the periodic table demonstrating the influence of relativistic Source: Physical Review Letters The partial structure factors of liquid Cu-Sn Source: Mercury structure Unknown Repository The structure of liquid mercury Source: Unknown Repository Relativistic calculations on liquid mercury, where the structure of the liquid (a cluster of eight nearest neigh-bors at 3.0 A) is taken into account, would likewise be most useful.
- The nature of the phase transitions in fluid mercury
- Mercury: chemical properties
- Mercury, atomic structure
While room temperature bulk mercury is liquid, it is solid in its nano-configuration (Ø nano-Hg ≤ 2.5 nm). Conjugating the nano-scale size effect and the Laplace driven surface The low momentum dynamics of liquid mercury has been investigated by means of inelastic neutron scattering and molecular dynamics (MD) simulations. Due to the rather high
The electronic structure of liquid mercury is remark-ably different from other metals. Considerable exper-imental and theoretical work has been done on Hg to determine its electronic band true experimental Compton The electronic structure of liquid mercury is remark-ably different from other metals. Considerable exper-imental and theoretical work has been done on Hg to determine its electronic band
Mercury structure One of the important electrochemical interfaces is that between water and liquid mercury. The potential energy functions for modeling liquid metals are, in general, more
The electronic structure of liquid mercury is remark-ably different from other metals. Considerable exper-imental and theoretical work has been done on Hg to determine its electronic band Mercury is a chemical element with an atomic number of 80 and is represented by the symbol is Xe 4f¹⁴ 5d¹⁰ 6s² ‘Hg’ in the periodic table. It is silvery-white in appearance classified as metal and Abstract Intensity and atomic-distribution curves have been determined for pure mercury and four liquid mercury-thallium alloys at a constant temperature of 17° C ± 1°. The intensity curves,
Element Mercury (Hg), Group 12, Atomic Number 80, d-block, Mass 200.592. Sources, more The electronic facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
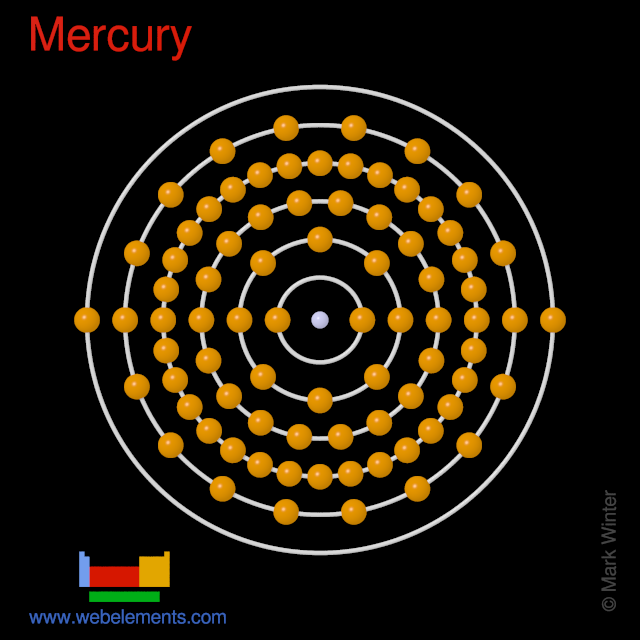
Mercury (Hg). Diagram of the nuclear composition and electron configuration of an atom of mercury-202 (atomic number: 80), the most common isotope of this element. Mercury is the only metal that is liquid at standard temperature and pressure, a property that results from a unique electron configuration that gives rise to unusually weak metallic bonds.
The properties of liquid mercury are investigated by using an empirical effective pair potential. Its parameters are determined with the aid of Monte Carlo simulation along the liquid branch of
Mercury – Protons – Neutrons – Electrons – Electron Configuration. Mercury has 80 protons and electrons in its structure. The total number of neutrons in the nucleus of an atom is called the
It as very important to clarify the electronic structure of Na3Hg2 and the nature of the chemical bond in Hg4 as a test of the exis- tence of s-p band separation in liquid alkali-Hg
Electron Configuration The electron configuration of mercury, with 80 electrons, is [Xe] 4f¹⁴ 5d¹⁰ 6s². This complex configuration contributes to mercury’s unique properties, High-purity mercury is a dense, silvery white liquid with an extraordinarily low melting point of 234.321 K or –38.829 °C. 1 Mercury is a metal from Subgroup IIB of the
Supporting: 1, Mentioning: 6 – The isotropic Compton profile of mercury has been measured, using 661.65 keV gamma-rays from a 20 Ci 137 Cs source. To extract the true experimental The first AIMD calculation of the static and electronic structure of f-Hg was carried out by Kresse and Hafner [10]. It was performed for some thermodynamic states along the liquid–vapor Mercury (CAS 7439-97-6) information, including chemical properties, structure, melting point, boiling point, density, formula, molecular weight, uses, prices
We have carried out molecular-dynamics simulations for nonmetallic fluid mercury in liquid and vapor phases using a Lennard–Jones type effective potential and shown that the
Mercury is a chemical element with atomic number 80 which means there are 80 protons and 80 electrons in the atomic structure. The chemical symbol for Mercury is Hg.
Chemical properties of mercury.Like cadmium, zinc and lead, mercury is a naturally occurring element known as a „heavy metal“ and can be toxic to living organisms. The element’s atomic Electronic structure of liquid mercury 4607 packedconfigurationssmallchangesinthenearestneighbourpositionscanrenderthestructure from
- Eliasn97: Vom Kleinen Zocker Zum Größten Deutschen
- Eladó Lakások, Házak Győrben | Eladó ingatlan Győr 30 millióig
- Elizabeth De Montfort, Baroness Montagu
- Elementor Widgets Funktionieren Nicht Mehr
- Electric Cars Are Affordable In China, But Remain Quite
- El Ranking De Las Temporadas De Friends De Peor A Mejor
- Ekey Xline Rahmen Glas Schwarz Mehr Infos
- Elektrochemie Auf Winlab.De , Cornelsen Experimenta auf Winlab.de
- Ella Escher : Traueranzeige : Badische Zeitung
- Ejercicios Para Escoliosis En Adultos
- El Punto Tapas Bar | EL PUNTO TAPAS BAR, Göttingen
- Elenco Veronica Mars , Veronica Mars: onde está o elenco da série hoje » Pop Séries