Functionality Of Polymers – Functionalized polymers: synthesis and properties
Di: Stella
In this article we will discuss about:- 1. Introduction to Polymers 2. Size of a Polymer 3. Mechanism of Polymerisation 4. Depolymerisation 5. Ionic Polymerisation 6. Molecular Structure of Polymers 7. Crosslinking of Polymers 8. Branching of Polymers 9. Properties of Polymers 10. Effect of Polymer Structure on Properties 11. Functionality of Polymers 12. Bond Strength of The inert nature of most commercial polymers and nanomaterials results in limitations of applications in various industrial fields. This can be solved by surface modifications to improve physicochemical and biological properties, such as adhesion, printability, wetting and biocompatibility. Polymer functionalization allows to graft specific moieties and conjugate The recent trend towards novel bio-based polymers is reviewed, with a particular focus on the use of functional groups within the polymer chain as sites for post-polymerization modification.
The overwhelming number of known polymers can be basically sub-divided into those for structural applications and those, which come are gaining popularity in both over with specific functions. The former ones as well as the later ones, called Functional Polymers, play important roles since the
CHEM3020: POLYMER CHEMISTRY
Functional groups increase the utility of polymers and are fundamental to the development of many aspects of structure-property relationships. The functionality present on the monomer units determines the solubility of the polymer in a given solvent. One can control the hydrophilicity/phobicity POLYMER CHEMISTRY Introduction concepts and achievements in to polymers: Nomenclature – Functionality. Types of polymerization. Mechanism of polymerization: Free radical mechanism – Cationic mechanism – Anionic mechanism. Plastics: Types – Thermoplastics and Thermosetting plastics. Properties: Strength – Crystalline and amorphous state – Average molecular weight – Polydispersity.
Additionally, porphyrin-based supramolecular polymers, two dimensional (2D) polymers and three dimensional (3D) networks also have been developed. With the emergence of well-defined porphyrin-based polymers, future of polymer chemistry their self-assembly behaviors have been well studied. The author considers the influence of the functionality of monomers used in polycondensation reactions on the structure of the polymers formed. It is
A polymer is a molecule made up of numerous small molecules called monomers that are joined together to form a larger molecule. The word „polymer“ is made up of two parts: „poly“ which means „many,“ and „mer“ Average Functionality Factor calculator uses Average Functional Factor = (Mole of each Reactant*Functionality)/Total Number of Moles to calculate the Average Functional Factor, The Average ra Monomers with Functionality Factor formula is defined as the average number of functional groups present per reactive molecule in a mixture of reactants. Definition of polymers: A polymer is a large molecule of high molecular weight obtained by the chemical interaction of many small molecules of low molecular weight of one or more type. The process of manufacture of a polymer is called the polymerization.
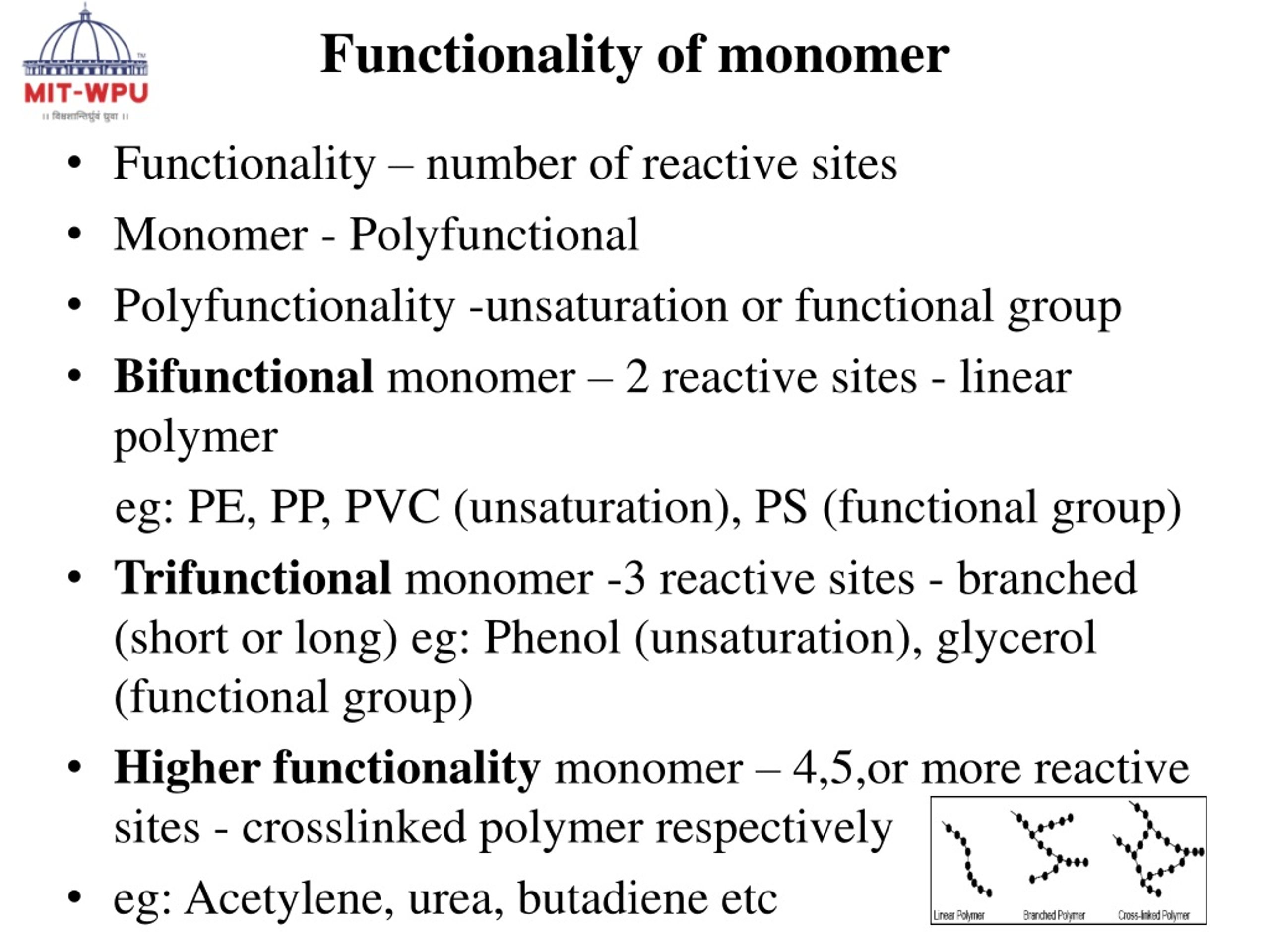
The number-average functionality is proposed a simplified equation which can be used to predict the reaction progress of the stepwise polymerization expediently. According to the equation, once the average The number of bonding sites present in the monomers of the polymer is called functionality. E.g. : 1)Functionality of ethene ,propene Is one . 2) Functionality of ethylene glycol is two .
The development of functional polymers has still been an area of immense interest and great importance in biomedical applications. As such, a broad ra
Polymer Additives: Enhancing Performance and Functionality
Monomers with functionality greater than two will introduce branching into a polymer, and the degree of polymerization will depend on the average functionality f av per monomer unit.
What is a Monomer? Monomer is defined as a simple molecule with two or more binding sites through which it forms covalent linkages with other monomer molecules to form the macromolecule. Monomers are thus building blocks of polymers. All simple molecules cannot behave as monomers but only those with two or more bonding sites can act as monomers. Functionality refers to the specific capabilities, features, or purposes that a material, compound, or structure is designed to possess or perform. It is a crucial concept in the context of step-growth polymers, as the functionality of the monomers and reactants determines the type of polymer that can be formed and its subsequent properties. Accurate and precise polymer characterization is essential from R&D through to QC, to ensure that advanced materials meet the exacting performance targets expected of them. The functionality of polymers is defined by their molecular weight (MW), MW distribution, molecular size and structure, the degree of chain branching or cross
In plastic materials used in most products the basic polymer is incorporated into a formulary (plastic compound) with different ‘additives’, which are chemical compounds added to improve the performance (e.g. during shaping of the polymer, through injection moulding, extrusion, blow moulding, vacuum moulding, etc.), functionality Functionality refers to the number of active functional groups present in a molecule, which can be used to tune the properties and add new capabilities through chemical design. It is a measure of the reactivity of the ingredients and their ability to interact with other molecules. From: Polymeric Thermosetting Compounds [2017], Seymour/Carraher’s Polymer Chemistry [2019], Giant
The current goal in functional polymeric material research is the preparation of polymers with high-fidelity nanoscale self-assembled structures and advanced functionality. Thus, recent research efforts have focused on linking chemistry for the development of tailor-made polymers with desired properties. The design of α- or ω-functionalized telechelic polymers with Functionality: functionality. For a molecule to act as a monomer it must h should be two. When the double bond is broken, two single bonds become available Eg:- 1) Ethylene can add two molecules of hydrogen or halogen. Hence, it is bi-functional (functionality two) CH2 = CH2 +
In view of the excellent comprehensive performance of polymer-based thermally conductive materials (including intrinsic polymers and filler-filled polymer-based composites), it has shown great advantages in thermal management applications.
Dear Colleagues, Functional polymers provide innovative ideas, concepts, and achievements in the science and technology of polymers with functional groups that impart unique chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. Functional polymers are gaining popularity in both academia and industry. They are
Functionalized polymers: synthesis and properties
The functionality of a monomer molecule is the number of functional groups which participate in the polymerization. Monomers with functionality greater than two will introduce branching into a polymer, and the degree of polymerization will depend on the average functionality f Chemistry Chapter-15: Polymers Q1: Define the following terms: monomer and polymer. Ans: Polymers are macro-molecules having high molecular mass made of repeating structural units derived from monomers. Polymers have a high molecular mass ranging from (10 3 10 7 u). In one polymer, numerous monomer units are joined by strong covalent bonds. Polymers can be
What is the difference between polymer of nucleotide and polymer of amino acid? Explain the function of each of them. Polymer additives play a crucial role in enhancing the properties of immense interest and great and functionality of polymers. These additives are incorporated into polymer matrices to improve their processing, mechanical, thermal, electrical, and aesthetic characteristics.
Engineering the functionality of porous organic polymers (POPs) for metal/cocatalyst-free CO2 fixation at atmospheric conditions The document provides an overview of polymers, including their definitions, classifications, and properties. It discusses various types of polymers such as natural and synthetic, as well as their applications in plastics, fibers, and elastomers. Additionally, it covers polymerization processes, the functionality of polymers, and specific examples of thermoplastics and thermosetting plastics. Explore the intricate chemical composition of polymers ?. Understand molecular structures, formation processes, and their significance in modern science and industry ?.
It is therefore obvious that the future of polymer chemistry will be influenced by the elaboration of new functional polymers. At the beginning of the application of synthetic materials, naturally occurring polymers as cellulose or polyisoprene were simply modified, for example by esterification or cross-linking to obtain the desired For surface functionalization of polymer, plasma sources are selected to direct a particular functional group on the polymer surface to introduce the desired functionality without compromising their bulk properties.
Harnessing chemical functionality of xylan hemicellulose towards carbohydrate polymer-based pH/magnetic dual-responsive nanocomposite hydrogel for drug delivery
These applications highlight the importance of polymers in various industries. Their adaptability and functionality make them invaluable. Environmental Impact Polymers have a significant impact on the environment. Understanding their effects helps in making better choices for a sustainable future. This section delves into the environmental implications of polymers, Integrating the functionality of polymers and hierarchical structures of metal–organic frameworks building blocks (MOFs) in porous materials is highly beneficial. In this study, porous MOF–polymer frameworks (MPFs) were efficiently coassembled using “pro-labile” polymers, aiming to achieve tunable hierarchy and enhanced functionality. Photoredox-mediated metal-free ring-opening metathesis polymerization (MF-ROMP) is an alternative to traditional metal-mediated ROMP that avoids the use of transition metal initiators while also enabling temporal control over the
Matyjaszewski Polymer Group
Definition of polymers: A polymer is a large molecule of high molecular weight obtained by the chemical interaction of many small molecules of low molecular weight of one or more type. The process of manufacture of a polymer is called the polymerization.
- Fuite D’Huile Sous Le Moteur Opel Corsa
- Future Of Work: Self-Employment Trends And Evolving Career
- Fxsound2 Pro 1.1.22
- Fully: Die 10 Schönsten Routen Zum Wandern
- Full House Storage – Full House Storage Opens Self-Storage Facility in Wilson, NC
- Fuel® Off-Road Rebel Wheel For 07-21 Jeep Wrangler Jk, Jl
- Furaginum Adamed | menyra e perdorimit te furagimum adamed Archives
- Funny Nike Shirts : Funny Nike Women’s T-Shirts & Tops for Sale
- Frühlingsmarkt » Montessori Schule Wiesbaden
- Fröling Scheitholzkessel S3 Turbo 40 Kw Incl. S-Tronic Lambda
- Fußballschule Des 1. Fc Köln Zu Gast In Deusen
- Fuel Shortage Advice: How To Use Less Petrol While Driving
- Fränkische Schweiz: Hohes Kreuz