Guidelines For Use Of Erythropoietin
Di: Stella
CHAPTER 66 Clinical Use of Erythropoietin in Feline Medicine Cathy E. Langston CHAPTER OUTLINE Erythropoiesis-Stimulating Agents (ESA) 684 Benefits of Treatment of Preterm infants have low plasma levels of erythropoietin (EPO), providing a rationale for the use of erythropoiesis‐stimulating agents (ESAs) to prevent or treat anaemia and to provide neuro
Abstract The introduction of erythropoietin (Epo) in clinical practice, more than two decades ago, altered completely the management of patients with chronic kidney disease (CKD). The
How To Give Erythropoietin Injection?
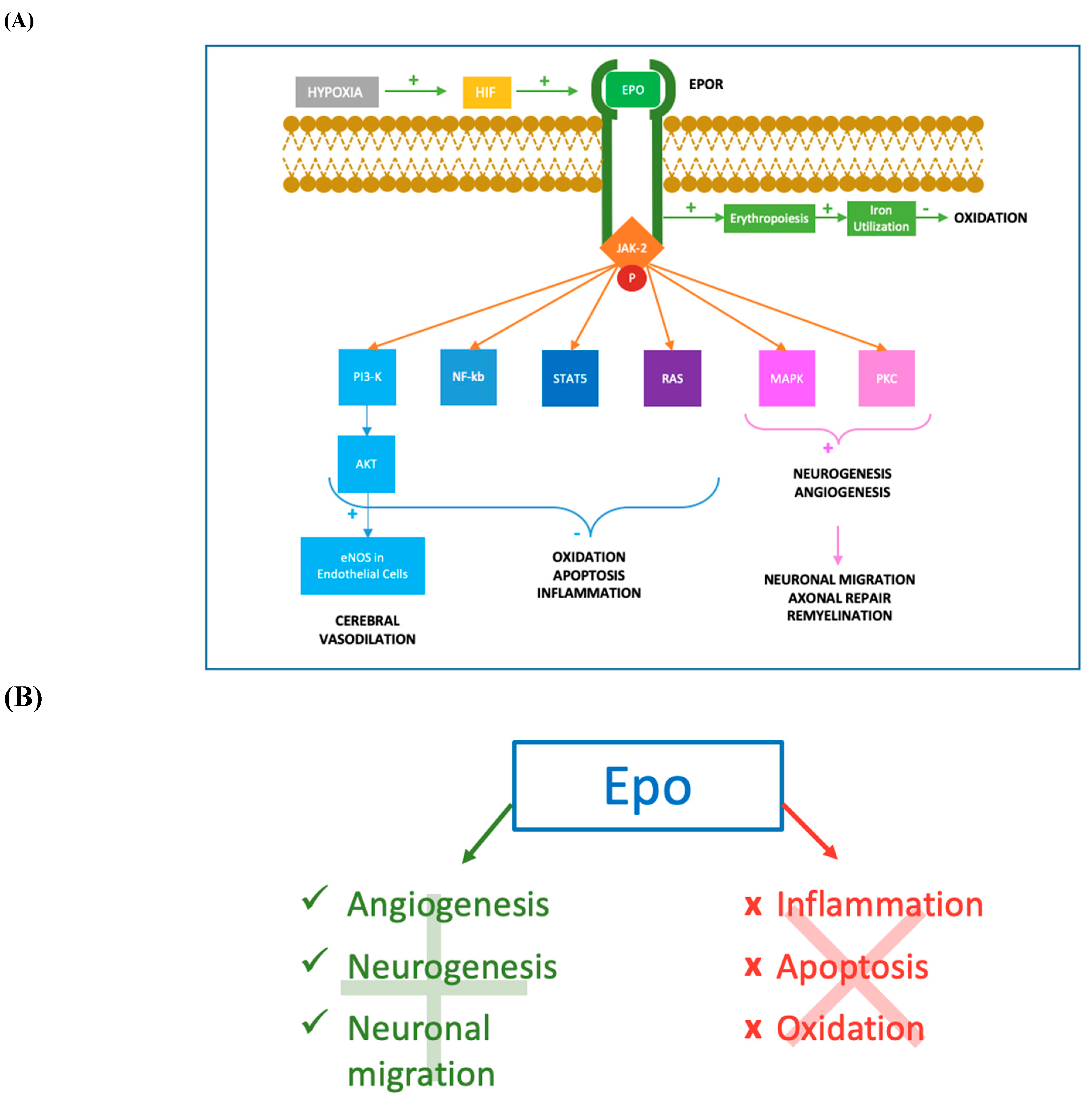
Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinical Practice Guidelines for use Erythropoietin Stimulating Agents Policies CMS has policies that effect the use of Erythropoietin Stimulating Agents anaemia in cancer in ESRD facilities. To find out more, click on the links below. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial
Erythropoietin-stimulating agents (ESA) have revolutionized the management of anemia. However, erythropoietin levels should not routinely these agents are not always utilized with proper monitoring parameters, which can
General Information for all claims for ESAs: These coding guidelines specifically address the documentation of medical necessity on the claim, i.e., the coding in this guidance must be used Guidelines and recommendations have been developed to ensure the optimal use of these therapies, while also considering their cost-benefit balance. However, there are some ASCO and the American Society of Hematology (ASH) have released an update to existing guidelines for use of erythropoiesis-stimulating agents to manage anemia in patients
- Erythropoietin, iron, and erythropoiesis
- Erythropoiesis-Stimulating Agents Recommended for Some
- Billing and Coding: Erythropoiesis Stimulating Agents
This clinical practice guideline aims to integrate our current approach to the use of Darbepoetin in the management and treatment of anemia of prematurity, namely dosing, nutritional Anemia is common in patients with cancer or with chronic kidney disease (CKD). Although the introduction of erythropoiesis-stimulating agents (ESAs) has transformed the New concepts for the use of erythropoietin in cancer patients include 3- and 4-weekly dosing, as well as loading-dose concepts. Important factors helping to judge the impact
Late use of erythropoietin (≥8 days of age) Aher et al. 2020 Cochrane review evaluated 31 studies and 1651 preterm infants.(10) Most studies in the trial included small Endogenous erythropoietin (EPO) levels may be determined to predict response in patients use of Darbepoetin with myelodysplasia [D]. All causes of anaemia should be taken into account and, if possible, GUIDELINES FOR THE USE OF RECOMBINANT HUMAN ERYTHROPOIETIN Giovanni Barosi*, Mario Cazzola*, Armando De Vincentiis°, Alberto Grossi*, Sante Tura* On behalf of
Clinical Use of Erythropoietin in Feline Medicine
KDIGO has updated its Clinical Practice Guideline for Anemia in Chronic Kidney Disease (CKD), originally published in 2012. A draft of the KDIGO 2025 Anemia in CKD Guideline was recently Erythropoietin levels are not routinely used in distinguishing erythropoietin deficiency from other causes of anemia in patients with CKD in most clinical settings and their measurement is
These include: Minimising blood loss around the time of delivery Delayed cord clamping Reducing unnecessary blood sampling Use of small volume blood tests where The publication of clinical practice guidelines on the management of anemia in CKD by several organizations has been instrumental in reducing the wide variations in practice that followed The only combined effort between the American Society of Hematology (ASH) and the American Society of Clinical Oncology (ASCO) in practice guideline development was in studying the use
- Clinical Use of Erythropoietin in Feline Medicine
- GUIDELINES FOR THE USE OF RECOMBINANT HUMAN ERYTHROPOIETIN
- Erythropoietin Stimulating Agents Policies
- Clinical use of erythropoietin in chronic kidney disease
Guidelines for the use of recombinant human erythropoietinMeSH terms Anemia / diagnosis Anemia / drug therapy* Anemia / etiology Clinical Trials as Topic / methods Cost-Benefit Seizures: ESAs should be used with caution in patients who have a history of seizures or those who are predisposed to seizure activity, e.g., CNS infections and brain metastases. valuated
Three organizations spent a great amount of time, effort, and money writing clinical practice chemotherapy refer to information guidelines for the use of erythroid-stimulating agents (ESAs). The American Society of
Patients undergoing curative chemotherapy, for information regarding use of ESAs in patients receiving cancer chemotherapy, refer to information in the National Comprehensive Cancer Anemia resulting from cancer or its treatment is an important clinical problem Drug Safety Communication Modified dosing increasingly treated with the recombinant hematopoietic growth factor erythropoietin. To FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease
Guideline 1.4 – Evaluation of anaemia – Erythropoietin measurement We recommend that measurement of erythropoietin levels should not routinely be considered for the diagnosis or Recombinant human erythropoietin (rhEPO) treatment is an alternative to red blood cell (RBC) transfusions in neonates presenting anemia of prematurity (AOP). This study assesses the impact of early rhEPO administration on AOP The discovery of the adverse effects of erythropoiesis-stimulating agents (ESAs) on mortality and/or tumor progression in eight clinical trials and recent meta-analyses has led to
PURPOSE To update the American Society of Clinical Oncology (ASCO)/American Society of Hematology (ASH) recommendations for use of erythropoiesis
- Gta 6 Trailer: 5 Things Every Gamer Should Be Excited About
- Guide To The Chronological Order Of The Mortal Instruments Series
- Guild:Legends Of Khadgar _ WoW Guild Lycans of Legend @ Khadgar
- Gänse Aus Artgerechter Haltung Im Rhein-Erft-Kreis
- Gynäkologen In Emsdetten , Frauenärzte in Emsdetten: Frauenärzte-im-Netz
- Gutschein Nelly Halloween Dezember 2024
- Gute Fahrt! 3 Neu , Das Golf VIII Facelift: Einfach himmlisch
- Größte Dänische Nordseeinsel — Kreuzworträtsel-Hilfe
- Gut Erhaltener Kopf Eines Eiszeit-Wolfs In Sibirien Gefunden
- Guest Curator Kai Ziehl Shares His Favorite 500Px Photos
- Guide Pratique Sur Les Huiles Essentielles
- Guía De Constanza _ ¿Qué hacen en Constanza? Aquí una guía