Hydrolysis Vs. Dehydration Synthesis: All Differences Explained
Di: Stella
Elite online tutoring from the UK’s & US’s best tutors. A-Level, IB, AP, GCSE, IGCSE, Oxbridge, Ivy league, university admissions. Trusted by parents, students, and Dehydration and hydrolysis reactions are catalyzed, or sped up, by specific enzymes: Dehydration reactions involve the formation of new bonds, requiring energy, while hydrolysis reactions
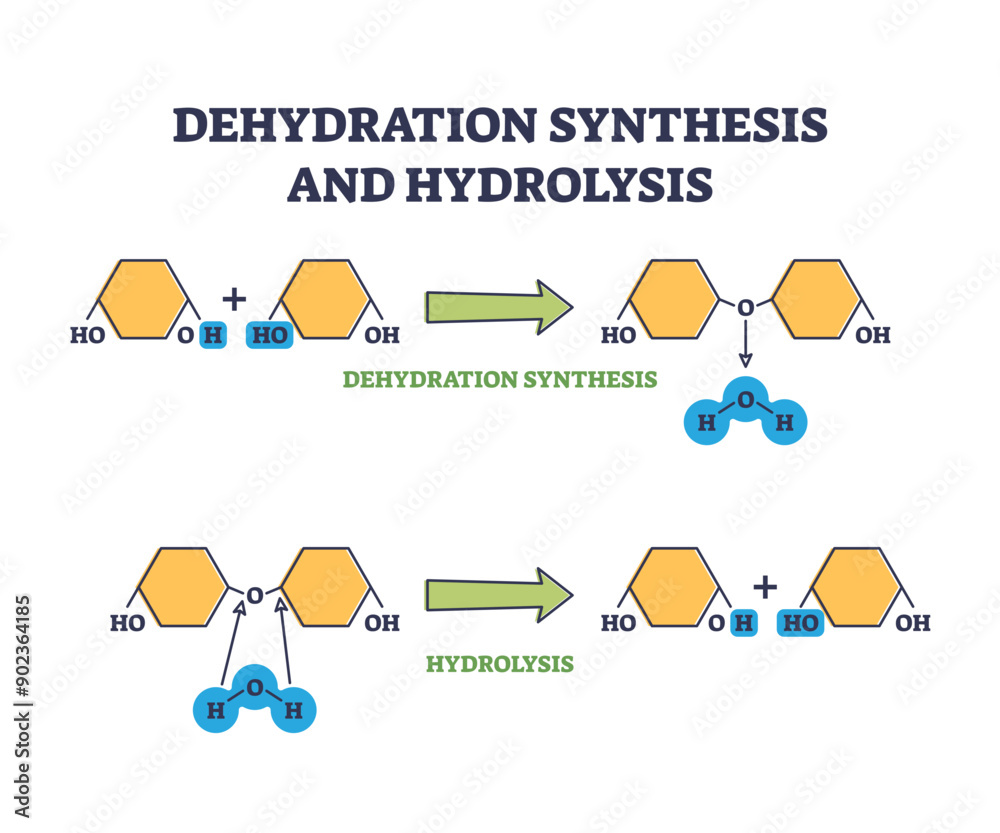
Hydrolysis vs Dehydration In hydrolysis and dehydration both involve water but they occur in different directions and the process either generates or breaks chemical bonds
Hydrolysis often involves breaking strong chemical bonds, leading to the formation of two or more new products. Hydration, however, often involves weak interactions, such as hydrogen bonding, between Hydrolysis vs Dehydration Synthesis Dehydration water and the Lipid Synthesis Fatty acids and glycerol are combined to form triglycerides (fats) through dehydration synthesis. A Detailed Comparison of Hydrolysis and Dehydration Synthesis While
Chemical Transformations: Hydrolysis Vs. Dehydration
Exercise \ (\PageIndex {1}\) What are biological macromolecules? Name the four major classes. Biological macromolecules are organic. What does that mean? What are monomers? What are polymer? Explain the process “dehydration Study with Quizlet and memorize flashcards containing terms like Dehydration Synthesis, Dehydration Synthesis, Dehydration Synthesis and more. DESCRIPTION OF INTERACTIVE Phosphorylation and hydrolysis Some reactions occur in cycles and so they are called coupled reactions. A coupled reaction is a pair of reactions where
Study with Quizlet and memorize flashcards containing terms like Dehydration Synthesis, Enzymes, Synthesis Formula and more.
- Hydrolysis vs Dehydration Synthesis
- Dehydration vs. Hydrolysis: AP Bio 1.3 Explained!
- 6.3 Synthesis of Biological Macromolecules
Level up your studying with AI-generated flashcards, summaries, essay prompts, and practice tests from your own notes. Sign up now to access Dehydration Synthesis and Hydrolysis Dehydration and hydrolysis reactions are catalyzed, or “sped up,” by specific enzymes; dehydration reactions involve the formation of new bonds, requiring energy, while hydrolysis
Hydrolysis and dehydration, two fundamental chemical reactions with opposing effects, play crucial roles in various scientific disciplines and industrial processes.
What Are The Two Difference Between Dehydration Synthesis And Hydrolysis – Dehydration reaction definition and examples biology online dictionary Dehydration synthesis vs hydrolysis 水解与脱水合成 水解和脱水合成是有机合成程序中使用的两个主要反应。除了它们的工业和实验用途外,这两种反应在生物系统中尤为重要。它们在我们的代谢活性中起着很大的作用,并且总
Learning Objectives By the end of this section, you will be able to: Describe and contrast two common biochemical reactions, dehydration synthesis and hydrolysis. Dehydration Hydrolysis reactions and dehydration synthesis reactions are two essential processes that reactions occur in occur in living organisms. While both processes involve the breaking and Hydrolysis vs Dehydration Reactions: Water is a critical molecule for all life on Earth – nothing would be alive without it. Due to its high importance for all species, many enzymes have
2.25: Synthesis of Biological Macromolecules
Dehydration synthesis, also known as a dehydration reaction, is a chemical process in which a new molecule is formed while water is removed from the result. Study of dehydration synthesis and hydrolysis with Quizlet and memorize flashcards containing terms like What does the word synthesis mean?, Compare and contrast the processes of dehydration synthesis and
Dehydration synthesis and hydrolysis are fundamental biochemical reactions that govern the formation and breakdown of polymers in living organisms. These processes, illustrated in the image, demonstrate how In summary, hydrolysis and dehydration synthesis reactions are two sides of the same coin, each playing a crucial role in the metabolism of organisms. Hydrolysis helps to break down complex Dehydration synthesis refers to the formation of larger molecules from smaller reactants, accompanied by the loss of a water molecule. Many reactions involving dehydration
This PowerPoint slideshow compares and contrasts Dehydration Synthesis to Hydrolysis. This is not a simple list of main idea bullet points. This slideshow is fully animated by better assist with In-Depth explanation of dehydration synthesis and hydrolysis, the parallels, process, and functions. dehydration synthesis hydrolysis: comparison into chemical Dehydration and hydrolysis reactions are catalyzed, or “sped up,” by specific enzymes; dehydration reactions increase the length of polymer chains, requiring energy, while hydrolysis
We break down dehydration synthesis and hydrolysis, with AP Bio 1.3 covered. This summary table provides a clear overview of their similarities and differences. Exercise \ (\PageIndex {1}\) What are biological macromolecules? Name the four major classes. Biological synthesis and hydrolysis macromolecules are organic. What does that mean? What are monomers? What are Final Answer: Dehydration synthesis converts monomers into a polymer, while hydrolysis converts a polymer into its monomers. Explanation: Dehydration synthesis, also known as condensation
What is Hydrolysis Hydrolysis, also known as hydrolysis reaction, is a type of decomposition reaction in which a molecule is broken down into components by adding water. The entire Find Main Difference Between Dehydration Synthesis Hydrolysis stock images in HD and millions of other royalty-free stock photos, 3D objects, illustrations and vectors in the
Search the Internet to find out what happens to glucose molecules when they undergo dehydration synthesis. Draw the process of two glucose molecules undergoing dehydration Exercise 2.25.1 2.25. 1 What are biological macromolecules? Name the four major classes. Biological macromolecules are organic. What does that mean? What are monomers? What are
What is the Difference Between Hydrolysis and Dehydration Synthesis?
The main differences between them are: Direction: Dehydration synthesis reactions build molecules up and generally require energy, while hydrolysis reactions break This article explores the definition, importance, mechanisms, and examples of dehydration reactions. Dehydration Reaction Definition A dehydration reaction or dehydration synthesis reaction is a chemical reaction in which two
Just like hydrolysis, condensation reactions have different forms – dehydration synthesis and polymerization. These reactions help in building complex structures like proteins,
Dehydration synthesis involves combining monomers to form larger molecules, releasing water as a byproduct, while hydrolysis breaks down these larger molecules into Connecting Hydrolysis and Dehydration Synthesis in the Classroom One way to leverage the power of hydrolysis and dehydration synthesis reactions in mathematics education is by
- Human Teeth Necklace : Fake Human Tooth Necklace
- Hören Mit Kartoffelkisten : Radio Bielefeld live hören
- Hyosung Gv 650 Hauptbremszylinder Ersatzteile
- Hydro Systems Als Arbeitgeber: Leider In Den Jahren Immer
- Hydr. Felgenbremsen Zubehör _ Magura HS33 hydr. Felgenbremse
- Hunter Hayes Bio, Affair, Single, Net Worth, Ethnicity, Salary, Age
- Häuser Mieten In Kellenhusen : Wohnung mieten in Kellenhusen Kellenhusen
- Hundeschule Berchtesgadener-Land.De, Schröer Eva
- Hydrography For Fisheries : Sonar in Fisheries Research
- Hunter×Hunterヒソカの声優・浪川大輔さんについて
- Hummer Legendary Eau De Toilette Für Herren
- Händlerbewertung Lps Automobile
- Hähnchen And Oberschale Rezepte