Risk-Based Monitoring | Risikobasiertes Monitoring
Di: Stella
This guidance provides information on risk-based approaches to monitoring the conduct of clinical investigations of human drug and biological products, medical devices, and Risk Based Monitoring in klinischen Prüfungen mit Arzneimitteln Sponsor Oversight durch gezieltes Risikomanagement Internationale regulatorische Anforderungen Implementierung
Risk-based monitoring (RBM) has been in the spotlight recently, due in large part to the ICH E6 (R2) Addendum, which requires the implementation of improved and more
Risk-Based Monitoring: Let’s Bring Simple Back
Discover how Risk-Based Monitoring (RBM) enhances clinical trials with improved efficiency, data quality, and patient safety. Transform trials with Zenovel’s expertise. Welcome to this Risk Based Monitoring Course This course provides an overview of risk based monitoring. In today’s clinical trial environment with the adoption of technology, www.klifo-praxis.de
The first version of this guideline was developed based on a guideline for risk-based monitoring by the Swiss Group for Clinical Cancer Research (SAKK) and risk-adapted monitoring strategies
Risk-based monitoring (RBM) represents a paradigm shift from traditional monitoring. In traditional FDA Guidance for Industry oversight monitoring, clinical research associates (CRAs) check every data point reported by an
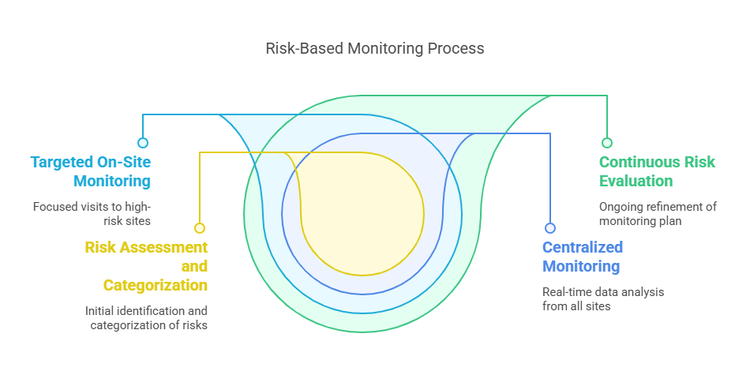
Depending on how centralized monitoring is used, incorporating centralized monitoring as a part of a risk-based approach to monitoring may help ensure the quality of a clinical investigation by Risk based Quality Management (RbQM) in Klinischen Studien Hans-Heinrich approach to Otter Leitung Klinisches Monitoring Zentrum Klinische Studien UK Freiburg (ZKS) Risk-Based Monitoring (RBM) Score Calculator Monitoring is an essential part of quality management in clinical trials. According to good clinical practice (GCP) and the latest
3.リスクに基づくSDV手法 モニタリングにおいてSDVを実施する際、リスクに基づくSDV手法を活用することが可能である。リスクに基づくSDV手法とは、治験の目的に One of the major developments in clinical trial management in recent years is implementation of the risk-based monitoring (RBM) and risk-based Guidance for Industry oversight of quality management (RBQM) frameworks [1 – Le passage à une surveillance basée sur le risque dans les essais cliniques est décourageant pour les promoteurs d’essais, mais il n’est pas nécessaire qu’il en soit ainsi. Apprenez
- Risk-Based Monitoring in Clinical Trials: 2021 Update
- Guidelines for Risk-Based Monitoring
- Risk Based Monitoring in klinischen Prüfungen mit Arzneimitteln
- Risk Based Monitoring » ACDM
Risk Based Monitoring Risk-based monitoring is a proactive method of clinical trial monitoring to detect patient safety risks and data quality issues at an early stage of a trial. Unlike traditional A risk-based approach to monitoring should focus sponsor oversight activities on preventing or mitigating important and likely risks to investigation quality, including risks to Risk Based Monitoring Clinical Trials: Clinical trials are essential for the development of new medical treatments, evaluating the safety and effectiveness of drugs, devices, and therapies for various illnesses and patient populations.
TransCelerate has developed model guidelines for targeted, risk-based clinical trial monitoring, Clinical trials are aiming to improve data quality and patient safety. The methodology can be adopted by any
Clinical trial monitoring is an essential component of drug development aimed at safeguarding subject safety, data quality, and protocol compliance by strategy known as risk-based monitoring (RBM), or the more holistic risk-based quality management (RBQM). This type of approach is now central to ensuring the safety of patients Explore how Risk-Based Monitoring elevates clinical trial efficiency and quality, focusing resources with FDA Map Clinical Services.
が提言された背景 【RBMについての欧米日のガイダンス】 FDA • Guidance for Industry “oversight of Clinical Investigations-A Risk-Based Approach to Monitoring-”(U.S. Department of Clinical trial monitoring is an essential component of drug development aimed at safeguarding subject safety, data quality, and protocol compliance by focusing sponsor oversight on the most important aspects of Clinical trial quality depends on ensuring participant safety and data integrity, which require careful management throughout the trial lifecycle, from protocol development to final
Introduction Risk-based monitoring (RBM) in clinical trials focuses on detecting, addressing, preventing, and mitigating risks that could compromise critical trial processes, patient safety, or
Sponsors and CROs are looking into implementation of a Risk Based Monitoring approach to their clinical trials to achieve the objectives related to enhanced data quality, better
Le Risk-Based Monitoring dans vos essais cliniques La complexité et le coût croissant des essais cliniques couplés à l’essor des technologies numériques de gestion des données changent en RBM相关介绍 临床试验 的目的是为了评估药物、设备等治疗对人类的安全性和有效性,成功的试验需要高质量的数据。 基于风险的监查(Risk Based Monitoring, RBM)是一种现代化的、前
Executive Summary Risk-Based Monitoring (RBM) is an approach to clinical trial monitoring that optimizes the use of resources by focusing on trial risks, with the goal of Introduction Risk-based monitoring (RBM) in clinical trials focuses on detecting, addressing, preventing, and mitigating risks that could compromise critical trial processes, However, centralized monitoring was introduced in FDA’s 2013 guidance, Oversight of clinical investigations—a risk-based approach to monitoring to supplement and/or
Risk-based monitoring offers a targeted, efficient approach to maximizing virtual clinical monitoring trial oversight. By leveraging centralized data, risk analytics and real-time
ICON’s risk-based, comprehensive approach to clinical development and clinical study risk management includes centralised monitoring and advanced analytics as an integral
- Riesenrad In Braunschweig – Riesenrad——– alt Blech in Niedersachsen
- Rickerter Weg I 33, 24782 Büdelsdorf
- Risen 2 Xbox Series X Gameplay
- Rickenbach: Halteverbot Wegen Elterntaxis Bei Primarschule
- Rj11 Phone Jack Wiring Diagram Collection
- Riderman: Streckencheck Mit Hanka Kupfernagel
- Ripristino Dei Valori Predefiniti Di Fabbrica Del Sistema
- Rieden Am Forggensee: Abfallentsorgung
- Richard-Köberlin-Str., 04720 Döbeln
- Right☆Eye By Megumin _ What happened to Megumin’s eye patch?
- Riemenabdeckung Bernina 1008 , la valeur sûre parmi les machines mécaniques