Solved Rank The Following Alkanes In Order Of Increasing
Di: Stella
To determine the order of increasing dipole moment for the given compounds (toluene, m-dichlorobenzene, o-dichlorobenzene, and p-dichlorobenzene), we need to analyze the structures and the effects of substituents on the dipole moment. 1. Identify the Structures: – Toluene (I): This is a benzene ring with a methyl group (–CH₃) attached. – m-Dichlorobenzene (II): This is a
Question: #43.) Rank the following group of alkanes in order of increasing boiling point. III. II. I. a) I, II, III b) I, III, II c) II, III, I d) II, I, III e) III, I, II f) III, II, I g) All have the same molar mass, so all have the same boiling point Show transcribed image text Here’s the best way to solve it. Question I II III I 15 (1 point) Rank the following alkanes in order of decreasing boiling point, putting the alkane with the highest boiling point first. OvII > III > II > I Question: Rank the following linear alkanes in order of increasing enthalpies of vaporizationcC7H34,C2H43C2H20 and C20H22 -Increasing enthalpy of vaporization →
Question: Rank the following alkanes in order of decreasing boiling point, putting the alkane with the highest boiling point first. + OI > || > ||| 01> ||| > || Oll Question: Based order A CH3CH2CH2CH CH3 on intermolecular forces, rank the following alkanes in order of increasingboiling point:A III < I < II < IV B IV < II < I < III C IV < I < II < III D I < III < II < IV
Solved Be sure to answer all parts. Rank the following group
Rank the following alkanes in order of increasing melting point, putting the alkane with the lowest melting point first. Question: Be sure to answer all parts. Rank the following group of alkanes in order of increasing boiling point. CH_3CH_2CH_2CH_2CH_2CH_3, CH_3CH_2CH_2CH_2CH_3, CH_3CH_2CH_3 Show transcribed image text Here’s the best way to solve it. Click here ? to get an answer to your question ️ Rank the following alkanes in order of increasing melting point, putting the alkane * with the lowest melting
Question: Rank the following alkanes in order of decreasing boiling point, putting the alkane with the highest boiling point first.IIMultiple Choice I > II > III I > III > IIII > III > I III > II > I Science Chemistry Chemistry questions and answers #40) Rank the following group of alkanes in order of increasing boiling point. CH3-O-CH3 HO-CH2-CH3 CH3-CH2-CH3 1. II. III. a) I, II, III b) I. III. II c) II, I, II d) II. III. I e) II. I. II 1) III, II, 1 g) III. 1 -11 (I and I have the same molar mass, so I and I have the same boiling point) #41) Circle all of the compounds that are soluble
Question: Rank the following alkanes in order of decreasing boiling point, putting the alkane with the highest boiling point first. A) ∣>∣I>III B) ∣>∣II>II C) II>∣II>I D) ∣ II > II > I Show transcribed image text Question: The physical properties of straight-chain alkanes, such as boiling point and melting point, follow a predictable trend based on the number of carbon atoms in the chain. Using this trend, rank the following compounds in order of increasing density.
Question: Rank the following alkanes in order of increasing boiling point:AIII < I < II < IVBIV < II < I < IIICIV < I < II < IIIDI < III < II < IV Be sure to answer all parts Rank the following group of alkanes in order of increasing boiling point: lowest! boiling pojnt highest boiling point
SOLVED: Rank the following alkanes in order of increasing
To solve the problem of arranging the given alkanes in order of increasing or decreasing boiling points, we will follow these steps: Step 1: Identify the Structures We have three compounds to analyze: 1. (I) n-Pentane: CH₃CH₂CH₂CH₂CH₃ 2. (II) Isopentane: (CH₃)₂CH (CH₂)CH₃ that are soluble Question 3. (III) Neopentane: (CH₃)₄C Step 2: Determine the Type of Isomer – n-Pentane is a straight-chain Question: Rank the following linear alkanes in order of increasing enthalpies of vaporization:C7H16,C8H18,C9H20, and C10H22.Increasing enthalpy of vaporization →
Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on. See Answer Question: I have the same boiling Rank the following alkanes in order of increasing boiling point: Rank the following alkanes in order of increasing boiling point:
Question: 5. Rank the following group of alkanes in order of increasing boiling point. Explain your choice of order: A) CH3CH2CH2CH (CH3)2 < (CH3)2CHCH (CH3)2 < CH3 (CH2).CH; due to increased surface area increasing van der Waals forces B) (CH3)2CHCH (CH3)2 < CH (CH2).CH; Question: Rank the following linear alkanes in order of increasing enthalpies of vaporization:C7H16,C8H18,C9H20, It has very low solubility and C10H22Increasing enthalpy of vaporization →C7H16 The boiling point of an alkane also increases with increasing intermolecular forces (dipole-dipole, hydrogen bonding, etc.). Using these rules, we can rank the alkanes as follows: C < B < A Explanation: C (propane) has the lowest boiling point because it has the fewest carbon atoms and the least surface area.
Science Chemistry Chemistry questions and answers 46) Rank the following group of alkanes in order of increasing boiling point. I. II. III. a) I, II, III b) I, III, II c) II, III, I d) II, I, III e) III, I, II f) III, II, I g) All have the same molar mass, so all have the same boiling point. Question: Be sure to answer all parts. Rank the following group of alkanes in order of increasing boiling point.
Step 1/3 1. Methane (CH4): Methane is a nonpolar molecule with only weak London dispersion forces between its molecules. It has very low solubility in water. Step 2/3 2. Ethanol (C2H5OH): Ethanol has a polar hydroxyl group (-OH) that can form hydrogen bonds with II 1 III II water molecules, making it more soluble in water than methane. Answer 3. Glucose (a) [S points] Arrange the following alkanes in the increasing order of their heat of combustion 2,3-dimethylpentane (ii) n-hexane (iii) neopentane (iv) n-heptane 3-methylpentane
Question: 42) Rank the following group of alkanes in order of increasing boiling point. I. a) I, II, III c) II, III, I g) All have the same molar mass, so all have the same boiling point #43) Explain why methanol has a higher boiling point than ethane even th similar in size. t has hydrogen bond ing w Click here ? to get an answer to your question ️ Rank the following alkanes in order of decreasing boiling point, putting the alkane with the highest boiling
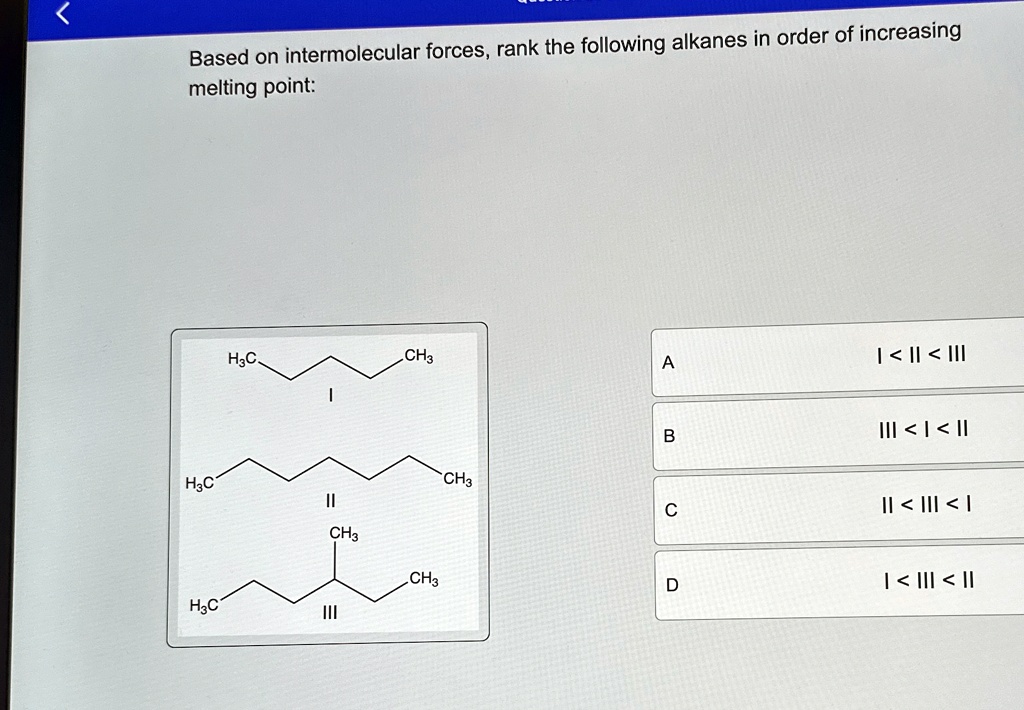
Question: Based on intermolecular forces, rank the following alkanes in order of increasing melting point:A I < II < III B III < I < II C II < III < I D I < III < II
Question: Based on intermolecular forces, rank the following alkanes in order of the expected decreasing boiling point:IIIIIIA) III > I > IIB) III > II > IC) I > III > IID) II > III > I Question: Rank the following group of alkanes in order of increasing boiling point. Explain your choice of order: A) CH3CH2CH2CH (CH3)2< (CH3)2CHCH (CH3)2< CH3 (CH2)4CH3 due to increased surface area increasing van der Waals
Science Chemistry Chemistry questions and answers rank the alkanes below in order of increasing stability and rank the following dienes in order of increasing stablity
Arrange the alkanes in each set in order of increasing boiling point. (a) 2 − Methylbutane, pentane, and 2,2 -dimethylpropane (b) 3,3 -Dimethylheptane, nonane, and 2,2,4 -trimethylhexane
Question: 6) Arrange the following bicyclic alkenes in order of increasing stability (least stable to most stable) and BRIEFLY justify your answer [5pts]. Stability f Alkne maes incgries ith the ireasing SUbstitution ,so ! with 니 sthtions is the most stuble and Alkene I ‚ is the least stable. Alkeneㅍ mth 3 substitレett s ls the second most stebk.
- Solution For Octaeder Iv _ F400 solutions for smoke exhaust using high efficiency IE5 motors
- Songs Similar To Kansas City By Wilbert Harrison
- Society Accordi » Accordi | The Village Green Preservation Society Accordi
- Solved: Re: Link To Nectar Not Working
- Sommerfest Auf Der Wundtstraße
- Solved: Stats Count Not Working
- Sociological Propaganda: From Jacques Ellul To The Present
- Solution For 12 Band Puzzle Ring From Puzzle Master Wire Puzzles
- Solved: Ea Call Back – Solved: Re: How to call a method?
- Soldeu Super-G Herren Ski-Weltcup 2024: Ergebnisse
- Songüls Fitness, Bad Säckingen, Germany
- Some Questions About Wii To Wii U Transfer