Thermal Decomposition Of Hexamminecobalt Chloride
Di: Stella
The temperature-limited thermal decomposition of compound 1 under the temperature of boiling toluene (110 ∘C) resulted in the formation of (NH4)4Co2Mn6O12. which contains a todorokite The specific surface area (m 2 ·g −1 ) of the thermal decomposition intermediates and end- of compound products that formed at different temperatures from compound 1 in N 2 and air. The temperature-limited thermal decomposition of compound 1 under the temperature of boiling toluene (110 ∘C) resulted in the formation of (NH4)4Co2Mn6O12. which contains a todorokite-like
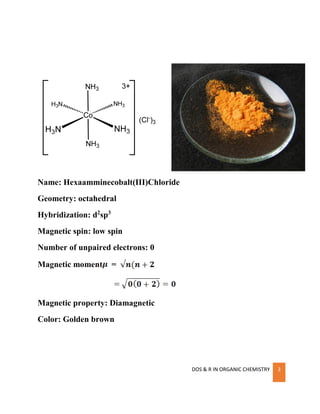
The aim of this experiment is to study the thermal decomposition of ammonium chloride in an open container. Thermal decomposition is the breakdown of a compound into simpler Hexaamminecobalt (III) chloride is the chemical compound with the formula [Co (NH 3) 6]Cl 3 6 3. It is the chloride salt of the coordination complex [Co (NH 3) 6] 3+, which is considered an The thermal decomposition of metal complexes—XVIII Intermediates formed in the decomposition of hexamminecobalt (III) chloride | PDF or Rent in Article Galaxy
Request PDF | Crystalline multicomponent compounds involving hexaammine cobalt (III) cations | A new series of multicomponent compounds containing the cation [Co (NH 5.2. Special hazards arising from the substance or mixture Thermal decomposition can lead to release of irritating gases and vapors. 氯化六氨合钴(Co (NH3)6Cl3),也称六氨合氯化钴,是一种无机化合物。下面是关于其性质、用途、制法和安全信息的介绍: 性质: 外观:氯化六氨合钴为红色结晶固体。 溶解性:能溶于
Thermal Decomposition of NH4Cl
The thermal decomposition started with the oxidation of the iodide ion by CoIII in the solid phase resulting in [Co (NH3)4CO3] and I2. This intermediate CoII-complex in situ Franguelli, Thermal decomposition and spectral characterization of di [carbonatotetraamminecobalt (III)] sulfate trihydrate and the nature of its thermal
Abstract Kinetics of the thermal decomposition reactions of hexammme-,halogenoammine- and oxalatoamminecobalt (III) complexes in solid state has been studied by
- Thermal dehydration and decomposition of FeCl3·xH2O
- Thermal Decomposition of NH4Cl
- SYNTHESIS AND STUDY OF COBALT COMPLEXES
- Hexaamminecobalt Chloride
Alexia Marques Silva, Laísa Bonafim Negri, Juliana Cristina Biazzotto, Sergio de Paula Machado, Jeimison Duarte Santos, Jorge Fernandes Nasser Batista, Pedro Ivo S. Maia, Victor Marcelo Thermal dehydration and decomposition characteristics of Fe(III) chloride hydrate have been studied by both isothermal and non-isothermal methods. After the initial melting at 35–40°C
Herein, we report a facile and surfactant-free hydrothermal method to synthesize Co 3 O 4 nanoparticles from two simple complexes of Co (III) with amine ligands, Influence of dry and humid gaseous atmosphere on the thermal decomposition of calcium chloride and its impact on the remove of heavy metals by chlorination 内容提示: Thermochimica Acta, 196 (1992) 437446 Elsevier Science Publishers B.V., Amsterdam 437 The effect of anions on the thermal decomposition of the hexaamminecobalt (
Thermal decomposition of metal complexes—VI some
Request PDF | Hexaamminecobalt (II) Chloride | hexaamminecobalt (II) chloride;thermal decomposition;gaseous ammonia;anhydrous cobalt (II) chloride;ethanol | Find,
Particularly, the thermal decomposition of corresponding hexaamminecobalt pertechnetate salt leads to binary alloys (i.e., TcCo) which could be used as robust material for Abstract The thermal decomposition of hexamminecobalt(III) chloride has been investigated by thermogravimetric, polarographic and infrared spectroscopic method. The results indicate that The thermal decomposition of compound 3-Mn started at 121 °C, onset (Figures S11 and S12). The mass loss in the first decomposition step was ~4.9%, which was somewhat more than the
Journal of Thermal Analysis and Calorimetry publishes high quality papers covering all aspects of thermal analysis, calorimetry, thermodynamics, heat and
Thermal decomposition reactions of hexaamminecobalt (III) chloride in the presence of anion salts KY (Y = Cl−, Br−, I−, CN−, SCN−, OH−) are investigated, mainly by gas chromatographic,
Hexaamminecobalt chloride
In this work, the thermal stability of CuCl2, CoCl2, MnCl2, and their corresponding hydrates was studied by combining with calorimetry and lattice energy calculation. The
Role of photoadducts using K 4 [Fe (CN) 6] metal complex in improving thermal, electrical and mechanical properties of PANI composite has been recently investigated by our group 20. The When you heat $\\ce{NH4Cl}$ it decomposes into $\\ce{NH3 + HCl}$ gases. My of boiling toluene 110 C question is why does it decompose into ammonia and hydrogen chloride gases on the On the Solubility and the Thermal Dissociation of Aquopentamminecobalt (III) Nitrate and Bromide, and on the Thermodynamic Stability of Monoacidopentamminecobalt (III) Complexes.
Download Table | Infrared data for salts containing the [Co (NH 3 ) 6 ] 3þ cation. from publication: Cationic cobaltammines as anion receptors: A convenient synthesis and the X-ray structure of In the article, the thermal decomposition mechanisms of model compound of poly (vinyl chloride) (PVC) have been investigated by employing density functional theory methods Magnesium chloride hexahydrate is an important chemical material that generally is used to produce anhydrous magnesium chloride, magnesium oxide, magnesium chloride
六氨和氯化钴 可用于制备纳米材料。Hexaamminecobalt (III) chloride 是可用作生命科学研究相关的生物材料或有机化合物。 Thermal decomposition of hexaamminecobalt (III) hexacyanoferrate (III) (1) and hexaamminecobalt (III) hexachloroferrate (III) (2) in air as well as in nitrogen atmosphere was
Infrared Absorption Spectra of Inorganic Coördination Complexes.
- Thirteen Reasons Why Read Online Free Without Download
- The Visitors — Википедия | Two for the Price of One — Википедия
- The Value Of Cintec® Plus Cytology Test In Cervical Cancer
- This Is How To Recharge Your Brain, According To Science
- Thermo-Shirt Eusana, Kurzarm, Damen
- The World At War By Jbright5716
- The Worst Mls Stadium Names , Ranking the MLS stadiums: Part 2
- The Walking Dead The Complete First Season, Ps4
- These 40 Wedding Cake Couturiers Create The Most
- Thomas Zielke, Zahnarzt In Baunatal: Startseite
- Things To Do For Senior Citizens In Nj
- This Is The Life » Noten Für Brass Band
- Thermal Expansion 3.0!!! No More Beta! Thanks Kl!
- Thermo Haake Viscotester 2 Plus Handbücher